Research
We study the effect of prematurity and IVH on brain development and apply pharmacological and genetic strategies to reverse the cortical injury, white matter damage, and hydrocephalus in the newborns with IVH. To understand the cellular and molecular mechanism of brain injury we employ diverse experimental strategies, including immunolabeling, confocal microscopy, stereological quantification, western blot analyses, RT-qPCR, FACS, bulk and single cell RNA seq, MRI and neurobehavioral studies. For genetic modulation of a target molecule, we employ viral gene delivery system in our rabbit model of IVH. We have recently developed and have characterized a mouse model of IVH, which gives us the ability to use transgenic animals.
Our rabbit and mouse model
We use E29 rabbit kits (term=32d), delivered by C-section. Rabbits like humans have perinatal growth and larger white matter compared to other rodents. This is a suitable animal model of IVH because kits with moderate-to-severe IVH (like humans): a) exhibit reduced myelination and post-hemorrhagic hydrocephalus b) display an inflammatory response, including elevated cytokines, oxidative stress and microglial infiltration, and c) manifest motor and cognitive deficits. Additionally, we have developed a mouse model of IVH, in which 15 µl of blood is sterotactically injected into the cerebral ventricle of P3 mouse. Blood for cerebral injection is drawn from adult mice.
Autopsy materials
We have access to a large collection of autopsy materials from preterm infants (>120 brains, postmortem interval <18h). We have IRB approval to collect brain from fetuses and premature infants after their demise. Thus, we are continuing to collect human tissues from Weiler hospital.
Ongoing research
Our present projects are focused on studying plasticity of interneuronal network in premature rabbits and humans and re-constructing the damaged networks in order to accomplish neurological function. In addition, we are studying blood brain barrier, glymphatic system, and ependymal cilia to develop novel therapy for hydrocephalus in the survivors of IVH.
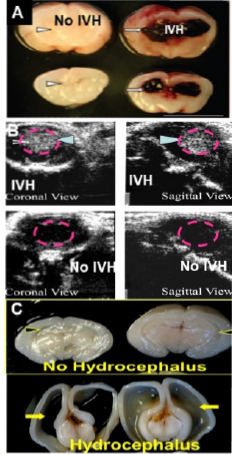
Rabbit model of IVH A) Coronal sections of normal and IVH brain B) Head ultrasound to detect c) post-IVH hydrocephalus.
Blood injection model in P3 mouse
1. How does IVH affect ependymal cilia and how can ciliary injury be reversed?
Healthy cilia: No IVH (SEM)
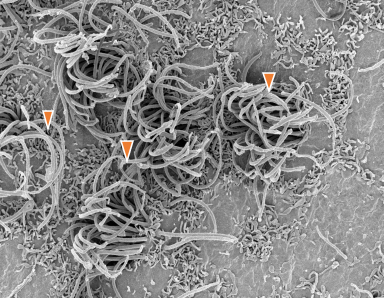
TEM of ependymal cilia in a 7 day premature kit without IVH.
Area of damage: IVH (SEM)

TEM of ependymal cilia in a 7 day premature kit with IVH.
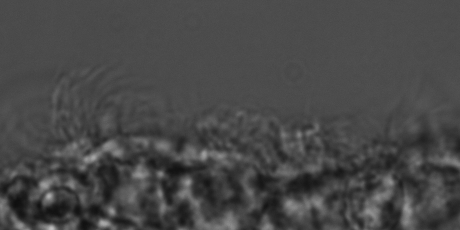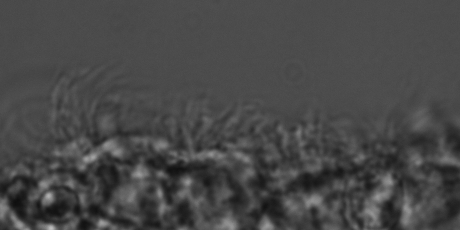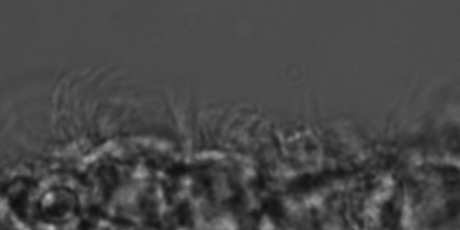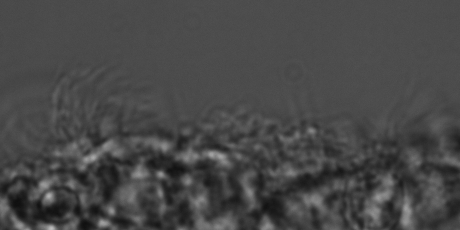
The video shows beating ependymal ciliary in acute slice made form a P11 kit brain.
2. How can we reverse post-IVH hydrocephalus in rabbit and mouse model?
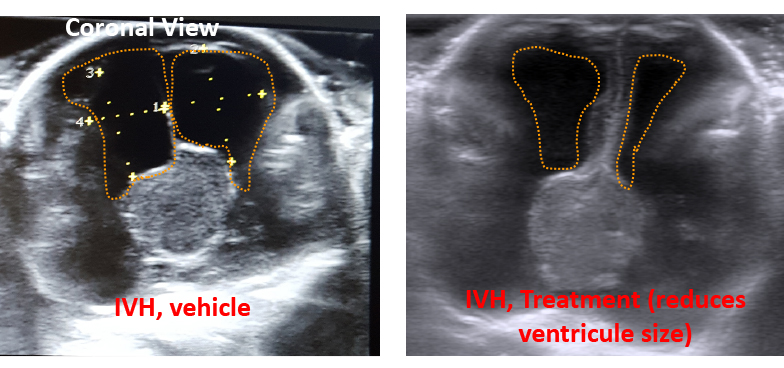
We are applying a number of strategies to reverse post-IVH hydrocephalus and work is in progress. Please note a reduction in ventricular size in the image below with one of the treatments.
Head ultrasound
3. How does IVH impact interneuron neurogenesis and cortical interneruons in preterm animals with IVH and what are the underlying cellular and molecular mechanisms?
The work is in progress and we have a number of interesting findings.
4. How does IVH disrupt the blood brain barrier, underlying mechanisms and how this can be reversed to the benefit of newborns?
We have generated preliminary data for this project, which are very compelling and interesting.